Bone Biomarkers in Mucopolysaccharidoses
Abstract
:1. Introduction
2. Physiology of Normal Bone Development and Remodeling
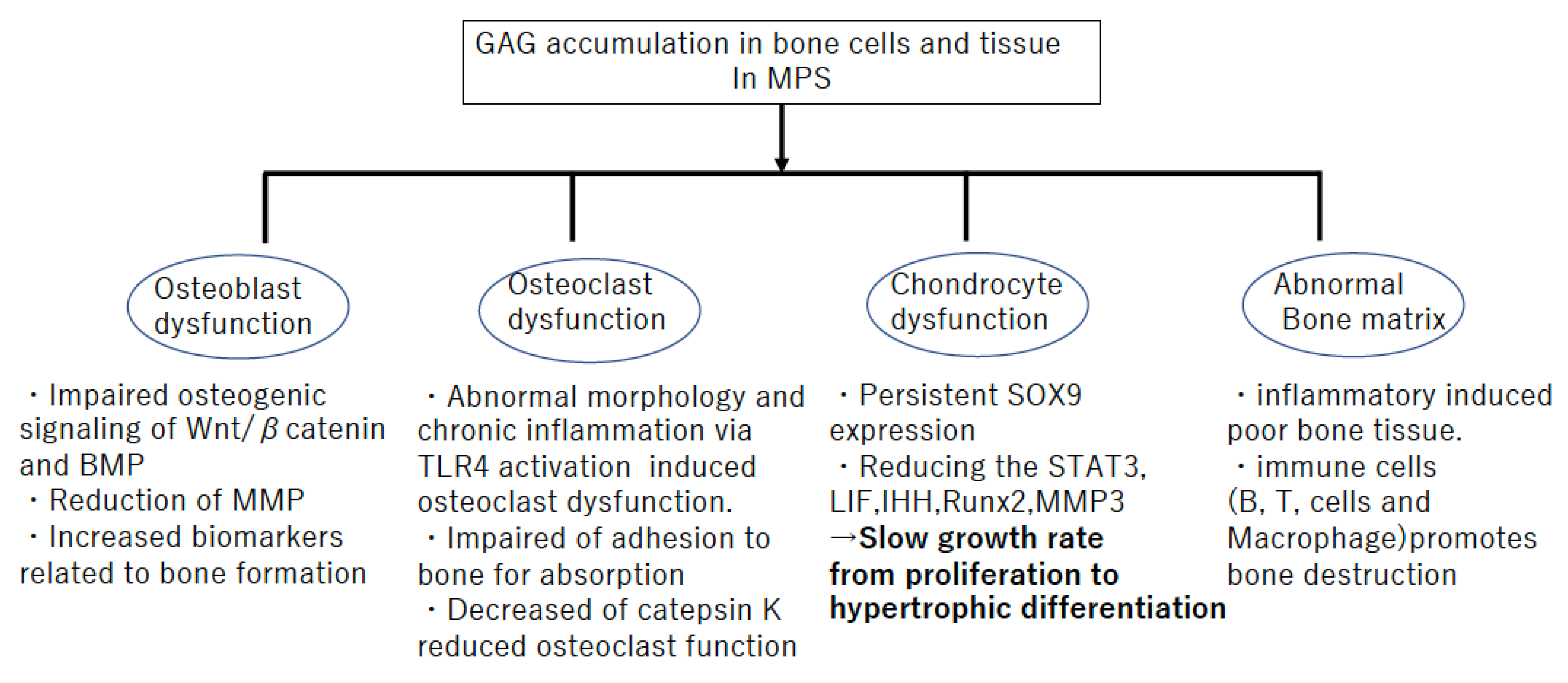
3. Pathophysiology Related to Bone Metabolism in MPS
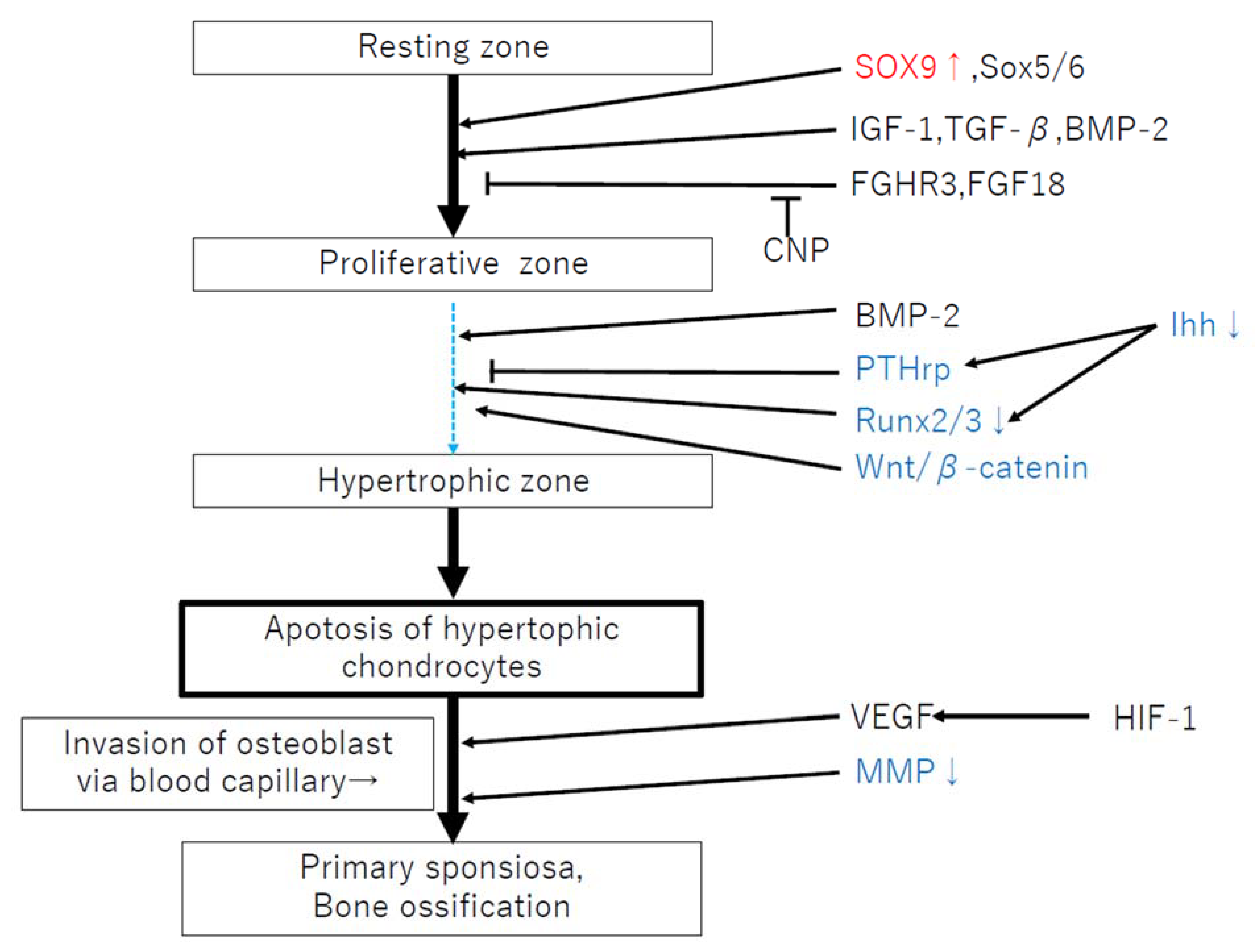
3.1. Accumulation of GAGs Impairs Chondrocyte Function
3.2. Failures of Endochondral Ossification
3.3. Accumulation of GAGs Lead to the Release of Inflammatory Cytokines via the Activation of TLR4
4. Biomarkers Related to Bone Metabolism in MPS
Funding
Conflicts of Interest
References
- Jiang, Z.; Lau, Y.K.; Wu, M.; Casal, M.L.; Smith, L.J. Ultrastructural analysis of different skeletal cell types in mucopolysaccharidosis dogs at the onset of postnatal growth. J. Anat. 2021, 238, 416–425. [Google Scholar] [CrossRef]
- Melbouci, M. Review: Growth impairment in mucopolysaccharidoses. Mol. Genet. Metab. 2018, 124, 1–10. [Google Scholar] [CrossRef]
- Aldenhoven, M.; Sakkers, R.J.B.; Boelens, J.J.; De Koning, T.J.; Wulffraat, N.M. Musculoskeletal manifestations of lysosomal storage disorders. Ann. Rheum. Dis. 2009, 68, 1659–1665. [Google Scholar] [CrossRef]
- Wraith, J.E. The clinical presentation of lysosomal storage disorders. Acta Neurol. Taiwanica 2004, 13, 101–106. [Google Scholar]
- Wraith, J.E. Lysosomal disorders. In Seminars in Neonatology; WB Saunders: Philadelphia, PA, USA, 2002; Volume 7, pp. 75–83. [Google Scholar]
- Valayannopoulos, V.; Nicely, H.; Harmatz, P.; Turbeville, S. Mucopolysaccharidosis VI. Orphanet J. Rare Dis. 2010, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroy, M.; Ross, F.; Teitelbaum, S.; Sands, M. Abnormal osteoclast morphology and bone remodeling in a murine model of a lysosomal storage disease. Bone 2002, 30, 352–359. [Google Scholar] [CrossRef]
- Rimoin, D.L.; Silberberg, R.; Hollister, D.W. Chodro-osseous pathology in the chondrostrophies. Clin. Orthop. Relat. Res. 1976, 114, 137–152. [Google Scholar]
- Nuttall, J.D.; Brumfield, L.K.; Fazzalari, N.L.; Hopwood, J.J.; Byers, S. Histomorphometric analysis of the tibial growth plate in e feline model of mucopolysacchatidosis type VI. Calcif. Tissue Int. 1999, 66, 47–52. [Google Scholar] [CrossRef]
- Russell, C.; Hendson, G.; Jevon, G.; Matlock, T.; Yu, J.; Aklujkar, M.; Ng, K.-Y.; Clarke, L.A. Murine MPS I: Insights into the pathogenesis of Hurler syndrome. Clin. Genet. 1998, 53, 349–361. [Google Scholar] [CrossRef]
- Silveri, C.P.; Kaplan, F.S.; Fallon, M.D.; Bayever, E.; August, C.S. Hurler syndrome with special reference to histologic abnormalities of the growth plate. Clin. Orthop. Relat. Res. 1991, 269, 305–311. [Google Scholar] [CrossRef]
- Wilson, S.; Hashamiyan, S.; Clarke, L.; Saftig, P.; Mort, J.; Dejica, V.M.; Brömme, D. Glycosaminoglycan-Mediated Loss of Cathepsin K Collagenolytic Activity in MPS I Contributes to Osteoclast and Growth Plate Abnormalities. Am. J. Pathol. 2009, 175, 2053–2062. [Google Scholar] [CrossRef] [Green Version]
- Ransfore, A.O.; Crockard, H.A.; Stevens, J.M.; Modaghegh, S. Occipito-atlanto-axial fusion in Morquio-Brailsfore syndrome. A ten-yeaar experience. J. Bone Jt. Surg. Br. 1996, 78, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.M.; Kendall, B.E.; Crockard, H.A.; Ransford, A. The odontoid process in Morquio-Brailsford’s disease. The effects of occipitocervical fusion. J. Bone Jt. Surg. 1991, 73, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Polgreen, L.E.; Thomas, W.; Fung, E.; Viskochil, D.; Stevenson, D.A.; Steinberger, J.; Orchard, P.; Whitley, C.B.; Ensrud, K.E. Low Bone Mineral Content and Challenges in Interpretation of Dual-Energy X-Ray Absorptiometry in Children with Mucopolysaccharidosis Types I, II, and VI. J. Clin. Densitom. 2014, 17, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Pastores, G.M. Musculoskeletal complications encountered in the lysosomal storage disorders. Best Pract. Res. Clin. Rheumatol. 2008, 22, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Le Douarin, N.M.; Smith, J. Development of the Peripheral Nervous System from the Neural Crest. Annu. Rev. Cell Biol. 1988, 4, 375–404. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Michigami, T. Current Understanding on the Molecular Basis of Chondrogenesis. Clin. Pediatr. Endocrinol. 2014, 23, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a Candidate Gene for Cleidocranial Dysplasia Syndrome, Is Essential for Osteoblast Differentiation and Bone Development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010, 339, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Baim, S.; Miller, P.D. Assessing the Clinical Utility of Serum CTX in Postmenopausal Osteoporosis and Its Use in Predicting Risk of Osteonecrosis of the Jaw. J. Bone Miner. Res. 2009, 24, 561–574. [Google Scholar] [CrossRef]
- Calvo, M.S.; Eyre, D.R.; Gundberg, C.M. Molecular Basis and Clinical Application of Biological Markers of Bone Turnover*. Endocr. Rev. 1996, 17, 333–368. [Google Scholar] [CrossRef]
- Eyre, D.R.; Koob, T.J.; Van Ness, K.P. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal. Biochem. 1984, 137, 380–388. [Google Scholar] [CrossRef]
- Hannon, R.; Blumsohn, A.; Naylor, K.; Eastell, R. Response of Biochemical Markers of Bone Turnover to Hormone Replacement Therapy: Impact of Biological Variability. J. Bone Miner. Res. 1998, 13, 1124–1133. [Google Scholar] [CrossRef]
- Hanson, D.A.; Weis, M.A.E.; Bollen, A.-M.; Maslan, S.L.; Singer, F.R.; Eyre, D.R. A specific immunoassay for monitoring human bone resorption: Quantitation of type I collagen cross-linked N-telopeptides in urine. J. Bone Miner. Res. 1992, 7, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Byers, S.; Casal, M.L.; Smith, L.J. Failures of Endochondral Ossification in the Mucopolysaccharidoses. Curr. Osteoporos. Rep. 2020, 18, 759–773. [Google Scholar] [CrossRef]
- Eyre, D.R.; Dickson, I.R.; Van Ness, K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem. J. 1988, 252, 495–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, E.; Cormier, C.; Steinmetz, P.; Kindermans, C.; Le Bouc, Y.; Souberbielle, J.-C. Differences in the Capacity of Several Biochemical Bone Markers to Assess High Bone Turnover in Early Menopause and Response to Alendronate Therapy. Osteoporos. Int. 2000, 11, 295–303. [Google Scholar] [CrossRef]
- Bellesso, S.; Salvalaio, M.; Lualdi, S.; Tognon, E.; Costa, R.; Braghetta, P.; Giraudo, C.; Stramare, R.; Rigon, L.; Filocamo, M.; et al. FGF signaling deregulation is associated with early developmental skeletal defects in animal models for mucopolysaccharidosis type II (MPSII). Hum. Mol. Genet. 2018, 27, 2262–2275. [Google Scholar] [CrossRef]
- Chiaro, J.A.; Baron, M.D.; del Alcazar, C.M.; O’Donnell, P.; Shore, E.M.; Elliott, D.M.; Ponder, K.P.; Haskins, M.E.; Smith, L.J. Postnatal progression of bone disease in the cervical spines of mucopolysaccharidosis I dogs. Bone 2013, 55, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Settembre, C.; Fraldi, A.; Jahreiss, L.; Spampanato, C.; Venturi, C.; Medina, D.L.; De Pablo, R.; Tacchetti, C.; Rubinsztein, D.C.; Ballabio, A. A block of autophagy in lysosomal storage disorders. Hum. Mol. Genet. 2008, 17, 119–129. [Google Scholar] [CrossRef]
- Tessitore, A.; Pirozzi, M.; Auricchio, A. Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI. Pathogenetics 2009, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Auclair, D.; Hein, L.K.; Hopwood, J.J.; Byers, S. Intra-Articular Enzyme Administration for Joint Disease in Feline Mucopolysaccharidosis VI: Enzyme Dose and Interval. Pediatr. Res. 2016, 59, 538–543. [Google Scholar] [CrossRef] [Green Version]
- Peck, S.H.; Lau, Y.K.; Kang, J.L.; Lin, M.; Arginteanu, T.; Matalon, D.R.; Bendigo, J.R.; O’Donnell, P.; Haskins, M.E.; Casal, M.L.; et al. Progression of vertebral bone disease in mucopolysaccharidosis VII dogs from birth to skeletal maturity. Mol. Genet. Metab. 2021, 133, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Baldo, G.; Wu, S.; Liu, Y.; Whyte, M.P.; Giugliani, R.; Elliott, D.M.; Haskins, M.E.; Ponder, K.P. Pathogenesis of lumbar spine disease in mucopolysaccharidosis VII. Mol. Genet. Metab. 2012, 107, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.J.; Martin, J.T.; Szczesny, S.; Ponder, K.P.; Haskins, M.E.; Elliott, D.M. Altered lumbar spine structure, biochemistry, and biomechanical properties in a canine model of mucopolysaccharidosis type VII. J. Orthop. Res. 2010, 28, 616–622. [Google Scholar] [CrossRef] [Green Version]
- Bartolomeo, R.; Cinque, L.; De Leonibus, C.; Forrester, A.; Salzano, A.C.; Monfregola, J.; De Gennaro, E.; Nusco, E.; Azario, I.; Lanzara, C.; et al. mTORC1 hyperactivation arrests bone growth in lysosomal storage disorders by suppressing autophagy. J. Clin. Investig. 2017, 127, 3717–3729. [Google Scholar] [CrossRef] [Green Version]
- Fraldi, A.; Annunziata, F.; Lombardi, A.; Kaiser, H.-J.; Medina, D.L.; Spampanato, C.; Fedele, A.O.; Polishchuk, R.; Sorrentino, N.C.; Simons, K.; et al. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J. 2010, 29, 3607–3620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pshezhetsky, A.V. Lysosomal storage of heparan sulfate causes mitochondrial defects, altered autophagy, and neuronal death in the mouse model of mucopolysaccharidosis III type C. Autophagy 2016, 12, 1059–1060. [Google Scholar] [CrossRef] [Green Version]
- Peck, S.H.; O’Donnell, P.J.; Kang, J.L.; Malhotra, N.R.; Dodge, G.R.; Pacifici, M.; Shore, E.M.; Haskins, M.E.; Smith, L.J. Delayed hypertrophic differentiation of epiphyseal chondrocytes contributes to failed secondary ossification in mucopolysaccharidosis VII dogs. Mol. Genet. Metab. 2015, 116, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, J.A.; Zhang, Y.; Hilton, M.; Long, F.; Ponder, K.P. Mechanism of shortened bones in mucopolysaccharidosis VII. Mol. Genet. Metab. 2009, 97, 202–211. [Google Scholar] [CrossRef]
- Andrade, A.C.; Nilsson, O.; Barnes, K.M.; Baron, J. Wnt gene expression in the post-natal growth plate: Regulation with chondrocyte differentiation. Bone 2007, 40, 1361–1369. [Google Scholar] [CrossRef] [Green Version]
- Church, V.; Nohno, T.; Linker, C.; Marcelle, C.; Francis-West, P. Wnt regulation of chondrocyte differentiation. J. Cell Sci. 2002, 115, 4809–4818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.Z.; Mak, K.K.; Taketo, M.M.; Yang, Y.Z. The Wnt/beta-Catenin Pathway Interacts Differentially with PTHrP Signaling to Control Chondrocyte Hypertrophy and Final Maturation. PLoS ONE 2009, 4, e6067. [Google Scholar] [CrossRef] [Green Version]
- Minina, E.; Kreschel, C.; Naski, M.C.; Ornitz, D.; Vortkamp, A. Interaction of FGF, Ihh/Pthlh, and BMP Signaling Integrates Chondrocyte Proliferation and Hypertrophic Differentiation. Dev. Cell 2002, 3, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Samsa, W.E.; Zhou, X.; Zhou, G. Signaling pathways regulating cartilage growth plate formation and activity. Semin. Cell Dev. Biol. 2017, 62, 3–15. [Google Scholar] [CrossRef] [Green Version]
- De Pasquale, V.; Pavone, L.M. Heparan sulfate proteoglycans: The sweet side of development turns sour in mucopolysaccharidoses. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 165539. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Derrick-Roberts, A.L.; Reichstein, C.; Byers, S. Cell cycle progression is disrupted in murine MPS VII growth plate leading to reduced chondrocyte proliferation and transition to hypertrophy. Bone 2020, 132, 115195. [Google Scholar] [CrossRef]
- Dy, P.; Wang, W.; Bhattaram, P.; Wang, Q.; Wang, L.; Ballock, R.T.; Lefebvre, V. Sox9 Directs Hypertrophic Maturation and Blocks Osteoblast Differentiation of Growth Plate Chondrocytes. Dev. Cell 2012, 22, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Simonaro, C.M.; D’Angelo, M.; He, X.; Eliyahu, E.; Shtraizent, N.; Haskins, M.E.; Schuchman, E.H. Mechanism of Glycosaminoglycan-Mediated Bone and Joint Disease: Implications for the Mucopolysaccharidoses and Other Connective Tissue Diseases. Am. J. Pathol. 2008, 172, 112–122. [Google Scholar] [CrossRef] [Green Version]
- De Vries-Bouwstra, J.K.; Goekoop-Ruiterman, Y.P.; Wesoly, J.; Hulsmans, H.J.; de Craen, A.J.; Breedveld, F.C.; Dijkmans, B.A.; Allaart, C.F.; Huizinga, T.W. Ex vivo IL1 receptor antagonist production upon LPS stimulation is associated with development of RA and with greater progression of joint damage. Ann. Rheum. Dis. 2007, 12. [Google Scholar] [CrossRef] [Green Version]
- Peck, S.H.; Tobias, J.W.; Shore, E.M.; Malhotra, N.R.; Haskins, M.E.; Casal, M.L.; Smith, L.J. Molecular profiling of failed endochondral ossification in mucopolysaccharidosis VII. Bone 2019, 128, 115042. [Google Scholar] [CrossRef]
- Taylor, K.R.; Yamasaki, K.; Radek, K.A.; Di Nardo, A.; Goodarzi, H.; Golenbock, D.; Beutler, B.; Gallo, R.L. Recognition of Hyaluronan Released in Sterile Injury Involves a Unique Receptor Complex Dependent on Toll-like Receptor 4, CD44, and MD-2. J. Biol. Chem. 2007, 282, 18265–18275. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Y.; Roehrl, M.H. Glycosaminoglycans are a potential cause of rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2002, 99, 14362–14367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Franceschi, L.; Roseti, L.; Desando, G.; Facchini, A.; Grigolo, B. A molecular and histological characterization of cartilage from patients with Morquio syndrome. Osteoarthr. Cartil. 2007, 15, 1311–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, T.C.; Doherty, T.M.; Eisengart, J.B.; Freese, R.L.; Rudser, K.D.; Fung, E.B.; Miller, B.S.; White, K.K.; Orchard, P.J.; Whitley, C.B.; et al. Biomarkers for prediction of skeletal disease progression in mucopolysaccharidosis type I. JIMD Rep. 2020, 58, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Mills, P.; Davison, J.; Cleary, M.; Gissen, P.; Banushi, B.; Doykov, I.; Dorman, M.; Mills, K.; Heywood, W.E. Free urinary glycosylated hydroxylysine as an indicator of altered collagen degradation in the mucopolysaccharidoses. J. Inherit. Metab. Dis. 2020, 43, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.A.; Kyle, R.; Alicia, K.; Ellen, F.; David, V.; Elsa, S.; Paul, J.; Orchard, C.; Chester, B.W.; Lynda, E.P. Biomarkers of bone remodeling in children with mucopolysaccharidosis types I, II, and VI. J. Pediatr. Rehabil. Med. 2014, 7, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Heywood, W.E.; Camuzeaux, S.; Doykov, I.; Patel, N.; Preece, R.L.; Footitt, E.; Cleary, M.; Clayton, P.; Grunewald, S.; Abulhoul, L.; et al. Proteomic Discovery and Development of a Multiplexed Targeted MRM-LC-MS/MS Assay for Urine Biomarkers of Extracellular Matrix Disruption in Mucopolysaccharidoses I, II, and VI. Anal. Chem. 2015, 87, 12238–12244. [Google Scholar] [CrossRef]
- Álvarez, J.; Bravo, S.; Chantada-Vázquez, M.; Barbosa-Gouveia, S.; Colón, C.; López-Suarez, O.; Tomatsu, S.; Otero-Espinar, F.; Couce, M. Plasma Proteomic Analysis in Morquio a Disease. Int. J. Mol. Sci. 2021, 22, 6165. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.G. Characterization and quantitative determination of the hydroxylysine-linked carbohydrate units of several collagens. J. Biol. Chem. 1969, 25, 602–612. [Google Scholar] [CrossRef]
- Fujitsuka, H.; Sawamoto, K.; Peracha, H.; Mason, R.W.; Mackenzie, W.; Kobayashi, H.; Yamaguchi, S.; Suzuki, Y.; Orii, K.; Orii, T.; et al. Biomarkers in patients with mucopolysaccharidosis type II and IV. Mol. Genet. Metab. Rep. 2019, 19, 100455. [Google Scholar] [CrossRef]
- Pinnell, S.R.; Fox, R.; Krane, S.M. Human collagens: Differences in glycosylated hydroxylysines in skin and bone. Biochim. Biophys. Acta (BBA)-Protein Struct. 1971, 229, 119–122. [Google Scholar] [CrossRef]
- Simonaro, C.M.; D’Angelo, M.; Haskins, M.E.; Schuchman, E.H. Joint and Bone Disease in Mucopolysaccharidoses VI and VII: Identification of New Therapeutic Targets and BioMarkers Using Animal Models. Pediatr. Res. 2005, 57, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Martell, L.; Lau, K.; Mei, M.; Burnett, V.; Decker, C.; Foehr, E.D. Biomarker analysis of Morquio syndrome: Identification of disease state and drug responsive markers. Orphanet J. Rare Dis. 2011, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brylka, L.; Jahnen-Dechent, W. The Role of Fetuin-A in Physiological and Pathological Mineralization. Calcif. Tissue Int. 2013, 93, 355–364. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Heiss, A.; Schäfer, C.; Ketteler, M. Fetuin-A Regulation of Calcified Matrix Metabolism. Circ. Res. 2011, 108, 1494–1509. [Google Scholar] [CrossRef]
- Eliyahu, E.; Wolfson, T.; Ge, Y.; Jepsen, K.J.; Schuchman, E.H.; Simonaro, C.M. Anti-TNF-Alpha Therapy Enhances the Effects of Enzyme Replacement Therapy in Rats with Mucopolysaccharidosis Type VI. PLoS ONE 2011, 6, e22447. [Google Scholar] [CrossRef] [Green Version]
- Seto, J.; Busse, B.; Gupta, H.S.; Schäfer, C.; Krauss, S.; Dunlop, J.; Masic, A.; Kerschnitzki, M.; Zaslansky, P.; Boesecke, P.; et al. Accelerated Growth Plate Mineralization and Foreshortened Proximal Limb Bones in Fetuin-A Knockout Mice. PLoS ONE 2012, 7, e47338. [Google Scholar] [CrossRef]
- de Boer, H.C.; Preissner, K.T.; Bouma, B.N.; de Groot, P.G. Binding of vitronectin-thrombin-antithrombin III complex to human endothelial cells is mediated by the heparin binding site of vitronectin. J. Biol. Chem. 1992, 267, 2264–2268. [Google Scholar] [CrossRef]
| Accumulating GAGs | Bone Finding in Previous Reports | |
|---|---|---|
| MPS I | Heparan sulfate, Dermatan sulfate | •Growth plate chondrocyte vacuolation •Failure of endochondral ossification [28] •Disorganized columnar structure [33] |
| MPSII | Heparan sulfate, Dermatan sulfate | •Failure of endochondral ossification [28] •FGF signaling deregulation during bone development [31] •Reduced calcification [28] |
| MPSIIIA | Heparan sulfate | •Reduced calcification [28] •Chondrocyte vacuolation [41] |
| MPSIVA | Keratan sulfate Chondroitin-6 sulfate | •Large, abnormal and vacuolated cartilage cells [28] •Failure of endochondral ossification [28] •Disorganized columnar structure [28] •Reduced calcification [28] |
| MPSVI | Dermatan sulfate Chondroitin-4 sulfate | •Growth plate chondrocyte vacuolation [34,35] •Failure of endochondral ossification [28] •Reduced calcification [28] |
| MPSVII | Heparan sulfate, Dermatan sulfate, Chondroitin-4 sulfate Chondroitin-6 sulfate | •Growth plate chondrocyte vacuolation [36,37,38,39] •Delay of development in primary and secondary centers of ossification [33] •Less ability to transit from the G1 to S phase and less ability to progress to mitosis or to exit the cell cycle [42] •Reduced length of limb bone and vertebrae [43] |
| Increased | Decreased | |
|---|---|---|
| Bone remodeling biomarkers | Osteocalcin, BSAP, urinary PYD, and DYD (MPS I, II, VI) [60] | |
| Pro-Inflammatory biomarkers | IL1β, IL6, TNF-α,TGF-β, NO, and EGF (MPSII, IVA) [59] MMP-1,MIP-1 and MMP-9 (MPS IVA) [57] | MMP-2 (MPS IVA) [61] |
| Transcriptional and genetic factors | SOX9 (MPS VII), COL2A1, NKX3-2 [42] | RUNX2, ALPL, BGLAP, COL10A1, osteopontin (SPP1) (MPSVII) [42] |
| Expressed protein biomarkers | MMP-2, -9,TIMP-1 (joint arthritis marker) [62] Lys-O-Gal/Lys-O-GalGlc (MPSI, II, IV) (collagen degradation marker) [63] | FOXA2, MMP13, PTCH1, PTH1R (MPS VII) [42] (chondrocyte maturation marker) TypeII A Collagen (MPSII, IVA) [64] |
| Other new biomarkersin each MPS | Collagen alpha-1 chain, Fatty acid-binding protein 5, Nidogen, cartilage oligomeric matrix protein, IGFBP7 (MPSI,IIneurological form) [61], βgalactosidase (MPSI, II neurological form, VI) [64] Glycosylated hydroxylysines (MPSIVA) A1T1, lipoprotein (a), SAA, clusterin and vitronectin (MPS IVA) [62] Protein HEG homolog1 (MPS VI) [64] | Fetuin-A (IVA) [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura-Utsunomiya, A. Bone Biomarkers in Mucopolysaccharidoses. Int. J. Mol. Sci. 2021, 22, 12651. https://doi.org/10.3390/ijms222312651
Nakamura-Utsunomiya A. Bone Biomarkers in Mucopolysaccharidoses. International Journal of Molecular Sciences. 2021; 22(23):12651. https://doi.org/10.3390/ijms222312651
Chicago/Turabian StyleNakamura-Utsunomiya, Akari. 2021. "Bone Biomarkers in Mucopolysaccharidoses" International Journal of Molecular Sciences 22, no. 23: 12651. https://doi.org/10.3390/ijms222312651






